In September 2025, the Draft International Standard (DIS) of the ISO 9001 revision was published. This formal step precedes the final publication of the new standard, which is scheduled for the end of 2026.
The review confirms changes with a direct impact on the quality governance of organizations. Among the main ones are strengthening the role of leadership and the culture of quality, explicit recognition of digitalization and data integrity, and the incorporation of aspects related to climate change and resilience in the organizational context.
This means that both companies are already certified under the standard, and those in the process of certification (or intending to achieve it) need to carry out immediate strategic planning to adapt to the expected changes in ISO 9001:2026.

What changes in ISO 9001:2026?
The revision of ISO 9001 is evolutionary in nature but introduces points that shift the focus and the type of evidence required from quality management systems. The essential topics for managers who want to ensure compliance with the standard are:
Leadership and culture
The new version places a clear emphasis on leadership: it highlights the active responsibility to promote a culture of quality, ethical behavior, and integrity as an integral part of governance. Top Management is expected to demonstrate commitment through decisions, communication, and performance evaluation.
Digitization & data integrity
The revision of ISO 9001 recognizes digital technologies (e.g., automation, AI, and integrated systems) as valid control and traceability tools without making them mandatory.
This highlights the importance of platforms that manage digital evidence in an immutable, auditable, and integrated way, such as a Quality Management System (QMS).
Climate change and sustainability
Aspects of environmental impact and resilience will be integrated into the organizational context. This forces companies to consider the climate risks and impacts of their operations (where applicable) to their quality scope and value chain.
Risk and supply chain
The new clarifications broaden the expectation on risk management, with special attention to third-party risks and controls over suppliers, from the selection of companies to the continuous evaluation of their performance. As such, supplier audits and the control evidence generated by them become critical to proving compliance with the new demands of ISO 9001.

Why does this matter to leaders and managers?
Updating is not only technical; it is also strategic. Risks of non-adaptation include: failures in certification audits, loss of competitiveness in purchasing processes (where customers require up-to-date compliance), reputational impacts, and potential contractual or regulatory implications.
On the other hand, a well-conducted transition transforms the QMS into a propeller of efficiency, risk reduction, greater operational predictability, and competitive advantage in front of customers and regulators. The final publication is scheduled for 2026, and the transition period for standard changes is up to three years – a period that should guide executive planning.
The risks of not adapting
- Failing audits and certifications: Failing to demonstrate evidence of compliance, data integrity, or leadership commitment can result in observations, non-conformances, or even loss of certification. This has a direct impact on contracts and access to markets.
- Tarnish company reputation and customer trust: Flaws in digital controls and supply chain processes are easy to expose and costly to repair. In companies that operate in regulated sectors, this can mean line stoppage or loss of strategic customers, in addition to negatively impacting the company’s reputation and image.
- Suffer contractual and regulatory sanctions: Customers and regulatory bodies tend to require up-to-date compliance with key standards (such as ISO 9001). Therefore, not meeting this demand can lead to fines, exclusion from purchase processes, or punitive contractual clauses.
- Operational ineffectiveness: Following the standards of the standard does not only bring compliance; they are also designed to generate efficiency and savings for companies. In other words, not doing this causes documentation to be dispersed and records to be questionable, which in turn generates rework, recall, and increased cost of non-quality.
The question that leaders and C-levels must ask themselves is not “do we have time to adapt?”, but rather “can we afford the consequences of not acting?”. The transition to the new ISO 9001:2026 is a time to transform obligation into differentiation. Leading this agenda means reducing risks today and gaining market share tomorrow.
Learn more: What is the role of Top Management in the new ISO 9001:2026?
How does SoftExpert Suite comply with ISO 9001:2026 changes?
Companies that seek to get ahead of the competition and ensure compliance with the new version of ISO 9001 need to combine efficient management, a solid strategy, and tools that are already prepared for these changes.
In the case of SoftExpert Suite, you have an all-in-one platform available in the cloud with interoperable modular functionalities and embedded artificial intelligence resources. This architecture favors the implementation of controls, evidence, and governance required by ISO 9001:2026. In other words, with just one system, you have all the tools needed to adapt to the new standard.
The SoftExpert Suite is designed to centralize processes, documents, audits, risks, and indicators in a repository with audit and reporting trails for Senior Management. That way, those in the operation have everything they need to work effectively, securely, and compliantly, and managers can track the operation in real time with reliable data and intuitive analysis tools.
In this way, the system automates and modernizes leadership, digitization, data integrity, risk management, supply chain visibility, and more.
| ISO 9001:2026 Requirement | SoftExpert Suite functionality | Benefits for your company |
| Quality leadership and culture. | Governance workflows, accountability matrices, and training/competency modules. | Evidence of commitment from Senior Management; documented decisions; measurable culture. |
| Data integrity/audit trails. | Immutable records and audit trails. | Robust digital proof in audits; Reduction of legal and compliance risks. |
| Risk management and supply chain | Risk and Supplier Management Module (for qualification and monitoring) | Mitigation of third-party risks; business continuity; improved supplier performance. |
| Audit and evidence | Audits Module (with checklists, attachments, evidence) | Reduced audit preparation time; Agile demonstration of compliance. |
| Metrics and governance | BI and executive dashboards | Strategic visibility; real-time monitoring and data-driven decision-making. |
| Skills and culture management | Employee training and assessment tools, such as Training and Competence | Ensure that employees are properly trained and aware of their role, transforming culture into something manageable and auditable. |
Quality management
The new version of ISO 9001:2026 remains the leading international quality management standard. This does not change with the latest update, but it does mean that traceability and automation will be increasingly relevant for companies looking to comply with the standard.
With the Enterprise Quality Management module present in SoftExpert Suite, you can manage non-conformities, CAPA, create action plans, and define and visualize quality-related indicators.
In this way, you automate flows, reduce rework, and provide historical logging for auditing through features such as:
- Registration and treatment of non-conformities with automated workflow.
- CAPA with assigned action plans, deadlines, responsible parties, automatic escalations, and a link between non-compliance and preventive corrective actions.
- Mapping of processes and indicators associated with the approval route for process changes.
- Link between incidents and suppliers, materials, and batches for investigation of their origin.
- Process management to analyze and model flowcharts, ensuring standardization and compliance with regulations.

Document and archive management
Traceability and digitization also need to be present in the document management of a company that wants to be ISO compliant — and, more than that, count on the benefits of this.
A company needs to ensure the integrity of its records and, in addition, demonstrate evidence ready and organized in audits. For this, it is necessary to perform version control, approvals, document lifecycle, evidence preservation, audit trail, and integrity of digital records. Remember: these are all explicit requirements of the new standard.
To facilitate compliance with these requirements, the Quality Management tool allows you to:
- Control document version and baselines, with approval and publication of workflows, as well as check-in/check-out.
- Edit metadata and document classification for quick search and regulatory hold.
- Traceability of changes by document (verifying who approved, when, what changes were made, among other data).
- Integration with electronic signature systems and the ability to export evidence packages.

Compliance audits (internal and external)
To succeed in an audit (whether internal or certification), it is necessary to have some basic elements: planning, execution, and digital evidence. This gives your company an easier way to demonstrate compliance.
By relying on SoftExpert Suite’s Audit tool, you reduce audit preparation time and increase the reliability of evidence, thus creating a controlled, data-driven process.
Plan, execute, and record evidence/demonstrations of compliance with automated checklists, evidence attachment, and other features such as:
- Planning internal/external audits with an audit program containing templates and checklists based on ISO 9001 clauses.
- Digital evidence attached to checklist items (such as images, reports, logs).
- Automatic registration of non-conformities detected with direct link to CAPA.
- Automatic reports for Top Management Review and exportable to the auditor of the accrediting entity.
Risk and supplier management
Through an integrated system, your company can link operational controls to strategic risks identified by Senior Management. This strengthens compliance, quality control, and even acts proactively in risk and failure management.
In the new version of ISO, this type of control also needs to happen over third parties (such as suppliers, for example). In addition, this control must also have visibility for Senior Management, which can thus make mitigation decisions.
By combining the GRC tool with the supplier management module , you perform the mapping and assessment of risks, define controls associated with them, and perform the qualification and continuous monitoring of suppliers. This is how it is possible:
- Record risks and create probability X impact matrices with mitigation plans and status tracking.
- Manage the supplier’s life cycle in all stages: registration, qualification, contracts, supplier audits, performance and periodic reassessment.
- Integrate events (e.g., non-compliance) and supplier score to perform automatic actions (such as partner suspension or requalification).
- View heatmaps and risk scenarios and filter by product, line or supplier.

Data analytics and internal governance
Finally, there is no point in having the right tools if there is no measurement of what is being done and, above all, the organization of information aimed at efficient decision-making.
The update of ISO 9001 places even more responsibility on the management of companies, which needs to act actively to ensure compliance and closely monitor the operation.
Through Analytics, leadership transforms data into evidence. Together with the Data Lab, you have automated dashboards and reports, and have the traceability of data and logs that support decisions and audits.
SoftExpert Suite’s Business Intelligence tools also bring:
- Pre-configured executive dashboards, with critical KPIs (such as CAPA time, percentage of critical non-conformities, supplier SLA, among others).
- Data drill-down and correlation between events (e.g., the relationship between suppliers, batches, and non-conformities).
- Scheduled and exportable reports with signature/health seals.
- Proactive trigger-based alerts.

8 steps for managers to adapt to the new ISO 9001:2026
To turn regulatory requirements into a competitive advantage, it’s not enough to just have the right tools — you also need to know how to implement and use them effectively.
This process involves protecting evidence, showing visibility to the board, and delivering quick wins that reinforce the added value of compliance with the updated ISO 9001. After all, the transition to the new version of ISO is a governance project, and therefore needs a strong PMO, clear metrics, and fast deliverables.
Below you can see a structure with the eight main steps that your company can take to facilitate this journey. But remember, there is no magic formula. Adapt everything to the reality of your company and the market in which it operates.
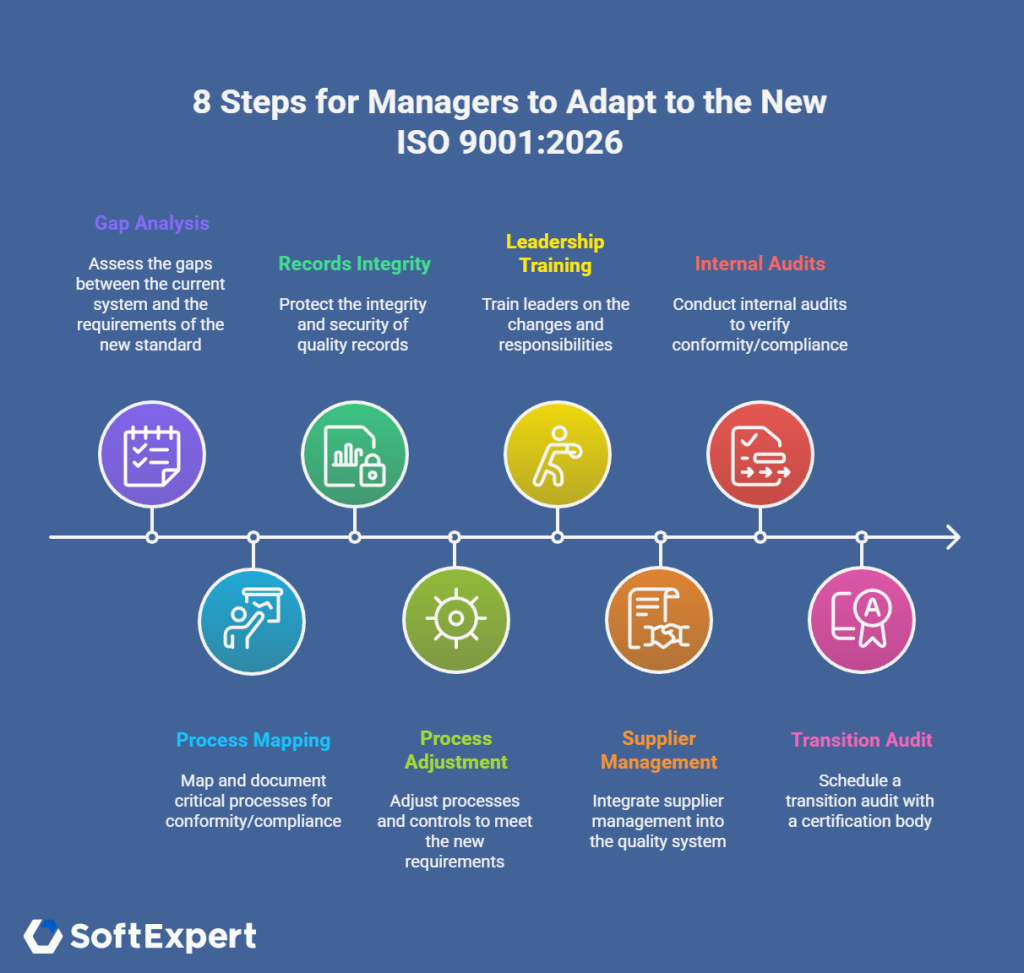
Priority actions (short-term)
- Do a complete gap analysis to identify how much your operation is in accordance with the standard. You can use the Requirement module of SoftExpert Suite to perform this task quickly.
- Map critical processes and document inventory. At this stage, you must identify where there is a lack of digital evidence and controls required by the standard.
- Protect log integrity with ECM configuration for versioning, retention, and audit trails.
Tactical actions (medium-term)
- Adjust processes and controls by creating CAPA templates and an executive dashboard for project monitoring. Automate critical flows to avoid failures and non-conformities.
- Train leaders on new responsibilities and governance indicators; also include the evaluation of ethical behavior in the executive routine.
- Integrate supplier management. To do this, define criteria for qualification, monitoring, and evidence, and monitor the performance of partners periodically.
Actions for certification (long-term)
- Organize internal audits to correct deviations and prepare digital evidence for ISO 9001:2026 certification.
- Schedule a transition audit with a certifying body within the period stipulated by the standard (the maximum period to adapt is three years after the final publication of ISO).
KPIs to track during the process
To make sure that each step of this transition is taking place properly and that ISO 9001 compliance follows your continuous improvement process, track metrics that show the health of the QMS and evidence of governance. The main ones are:
- percentage of critical non-conformities opened in the last 30 days;
- average time to CAPA closure (measured in days);
- percentage of records with full audit trail;
- percentage of qualified suppliers with active monitoring;
- Number of internal audits performed vs. planned;
- The compliance index indicated in the result of the audits (indicating the percentage of compliance for each clause of the standard).
Conclusion
ISO 9001:2026 brings clarifications and emphasis that make quality (and the evidence that supports it) more strategic than ever. For managers, the message is clear: acting early reduces risks, protects contracts and reputation, and transforms the QMS into a competitive advantage.
Integrated platforms such as SoftExpert Suite offer the set of capabilities — QMS, ECM, audits, risks, BI — necessary to respond quickly and securely to new requirements, ensuring traceability and executive governance.
If your company already uses digital systems, take advantage of this moment to validate the integrity of the evidence and map what needs to be updated; if the Quality Management System has not yet been digitized, the planning and adoption of an integrated solution should be a strategic priority.
Looking for more efficiency and compliance in your operations? Our experts can help identify the best strategies for your company with SoftExpert solutions. Contact us today!
FAQ – ISO 9001 2026 and SoftExpert Suite
The review emphasizes quality leadership and culture, recognizes digitization and data integrity, includes climate change/resilience impacts in the organizational context, and clarifies expectations about risk management and supply chain. All of this increases the evidence that the organization must provide in an audit.
Final publication is scheduled for 2026, and historically, transition windows have lasted up to 3 years after publication. This horizon should guide the schedule of actions.
Risks include audit observations or non-conformities, loss of certification, contractual and reputational impacts, fines/penalties, and increased costs of non-quality (such as recalls and rework).
It transforms the Quality Management System (QMS) into a level of efficiency (generating less rework), improves operational predictability, strengthens competitive advantage in bids, and reinforces trust with customers and regulators.
SoftExpert Suite centralizes processes, documents, audits, risks, and indicators in a repository with audit trails, workflows, and executive reporting, thus facilitating immutable evidence, traceability, and reporting for senior management.
The key module is the QMS, which has CAPA/non-conformities, document control and audit trails, audits with checklists and evidence, risk & supplier management, analytics with executive dashboards, and reports with integrity seal.
Prioritize conducting a gap analysis in your current QMS to align the culture of quality, ethics, risk management, and digitalization with the future requirements of the standard. This allows for a smoother and less disruptive transition.
Track indicators such as percentage of critical non-conformities opened in 30 days; average time of COVER closure; percentage of records with full audit trail; percentage of qualified suppliers with active monitoring, and number of internal audits performed vs. planned.
Top Management Review minutes, closed CAPA records with digital evidence, internal audit package with attachments, document inventory with versions and approvers, and risk matrix/supplier monitoring.
Compare costs avoided by incidents (recalls, fines, lost contracts) + hours saved with automation (CAPA, audit preparation) vs. program cost (licenses, services, FTE).